It has been beforehand reported that ursodeoxycholic acid (UDCA), a therapeutic bile acid, diminished danger for superior colorectal adenoma in males however not girls. Interactions between the gut microbiome and fecal bile acid composition as a consider colorectal most cancers neoplasia have been postulated however proof is restricted to small cohorts and animal research.
Using banked stool samples collected as half of a part III randomized medical trial of UDCA for the prevention of colorectal adenomatous polyps, we in contrast change in the microbiome composition after a 3-year intervention in a subset of individuals randomized to oral UDCA at 8-10 mg/kg of physique weight per day (n = 198) or placebo (n = 203).
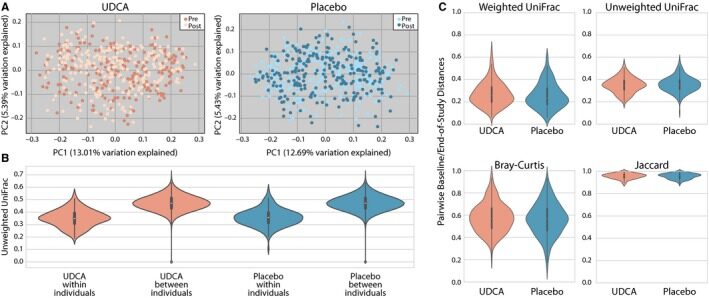
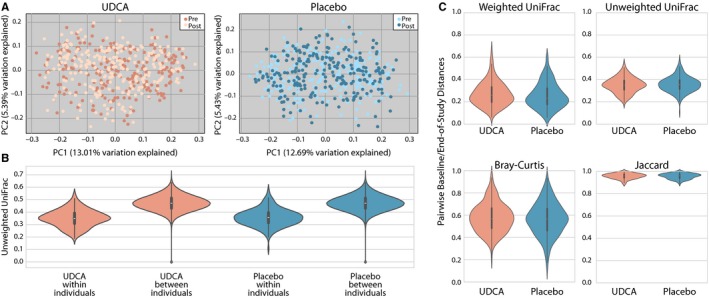
Study individuals randomized to UDCA skilled compositional modifications of their microbiome that had been statistically extra much like different people in the UDCA arm than to these in the placebo arm.
This mirrored a UDCA-associated shift in microbial group composition (P < 0.001), impartial of intercourse, with no proof of a UDCA impact on microbial richness (P > 0.05). These UDCA-associated shifts in microbial group distance metrics from baseline to end-of-study weren’t related to danger of any or superior adenoma (all P > 0.05) in males or girls. Separate analyses of microbial networks revealed an overrepresentation of Faecalibacterium prausnitzii in the post-UDCA arm and an inverse relationship between F prausnitzii and Ruminococcus gnavus.
In males who obtained UDCA, the overrepresentation of F prausnitzii and underrepresentation of R gnavus had been extra distinguished in these with no adenoma recurrence at follow-up in comparison with males with recurrence. This relationship was not noticed in girls.
Daily UDCA use modestly influences the relative abundance of microbial species in stool and impacts the microbial community composition with suggestive proof for sex-specific results of UDCA on stool microbial group composition as a modifier of colorectal adenoma danger.
Pharmacologically pertinent interval of impact (PPPE).
The interval of time throughout which a affected person is uncovered to a drug doesn’t essentially correspond to the interval throughout which the drug produces the opposed impact into consideration.
We suggest the time period Pharmacologically pertinent interval of impact (PPPE) to deal with this time window. We explored the PPPE in mild of the rofecoxib saga.We recognized the observational database research of rofecoxib at doses 25 and 50 mg each day and thromboembolic occasions. We additionally obtained the Kaplan-Meier curves of Vioxx Gastrointestinal Outcomes Research trial (VIGOR) and Adenomatous PolypPrevention on Vioxx (APPROVE) trials.We discovered seven observational research with 9 analyses.
All the research solely checked out present publicity. At the dose of 25 mg, solely three of 9 analyses had been barely statistically important. At the dose of 50 mg, the danger ratios had been a lot increased. The visible inspection of the Kaplan-Meier curves reveals that in the APPROVE trial (25 mg), the placebo and rofecoxib curves begin separating to turn out to be statistically considerably totally different solely after 36 months.
In distinction the VIGOR (50 mg), curves begin separating very early and the divergence will increase after Eight months.The 50 mg observational research, present publicity, correctively recognized the nearly speedy enhance in danger evident in the VIGOR Kaplan-Meier curves.
The absence of a right away enhance in danger proven by the APPROVE trial was additionally correctively recognized by most observational 25 mg research. To our information no observational research was finished on the long-term cardiac toxicity of the 25-mg dose. It would thus seem that the two doses of rofecoxib have totally different PPPEs.